過氧化氫作為一種可再生能源載體和清潔綠色的氧化劑,廣泛應(yīng)用于精細(xì)化工、生物制藥、環(huán)境修復(fù)等領(lǐng)域[1-3]。H?O?發(fā)生反應(yīng)只會產(chǎn)生O?和H?O反應(yīng)副產(chǎn)物,不會對環(huán)境造成環(huán)境污染[4,5]。
目前工業(yè)上最常見的生產(chǎn)H?O?的方法是蒽醌法,主要包括氫化、氧化、取代和循環(huán)四個步驟。雖然蒽醌法可以大規(guī)模生產(chǎn),但是能耗巨大,且需要使用有毒的有機(jī)原料和溶劑,會導(dǎo)致嚴(yán)重的環(huán)境污染,不符合綠色化學(xué)的發(fā)展要求[6-8]。因此,開發(fā)綠色H?O?制取方法具有非常重要的意義[9-11]。
近年來,越來越多的研究表明,通過光催化技術(shù)可以合成H?O?,且其原料僅為水和O?[12,13]。一般來說,光催化H?O?合成包括光吸收、電荷載流子的產(chǎn)生和分離、表面氧化還原反應(yīng)等多個步驟[14-18]。光催化生產(chǎn)H?O?具有環(huán)保性、高效性、靈活性、可持續(xù)性和創(chuàng)新性等多重優(yōu)勢,是一種具有廣闊應(yīng)用前景和可持續(xù)發(fā)展?jié)摿Φ男滦铜h(huán)保技術(shù)。
在光照條件下,光催化劑會產(chǎn)生許多光生電子-空穴對。位于光催化劑的導(dǎo)帶上的光電子具有較強(qiáng)的還原性,位于價帶上的光生空穴具有較強(qiáng)的氧化性[19]。光生電子驅(qū)動氧還原反應(yīng)(ORR)和光生空穴誘導(dǎo)水氧化反應(yīng)(WOR)都是重要的表面氧化還原反應(yīng),是光催化過氧化氫演化的主要原因(圖1)。
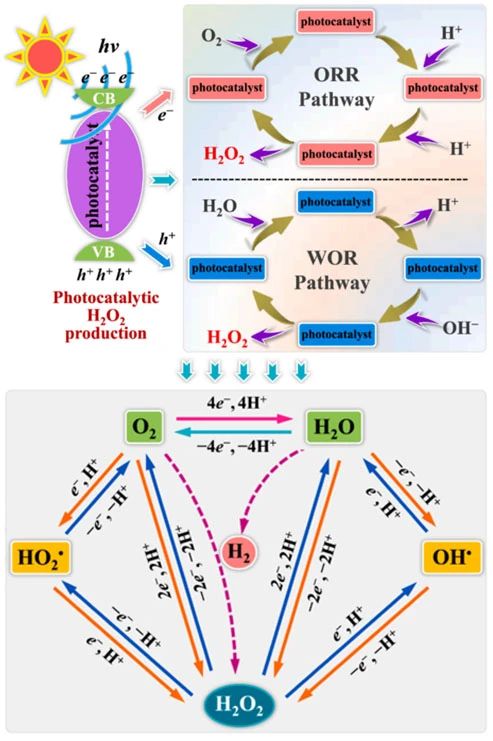
圖1.半導(dǎo)體上光催化H?O?合成示意圖
具體來說,光催化H?O?合成的兩種方式ORR和WOR,光催化劑在光照條件下產(chǎn)生光生電子和空穴,光生電子能夠?qū)?還原為H?O?,而光生空穴能夠?qū)?O氧化為H?O?[20-22]。如圖2所示,光生電子和空穴應(yīng)當(dāng)具有適當(dāng)?shù)难趸€原電位,才能夠滿足ORR和WOR反應(yīng)的發(fā)生條件[8,23]。
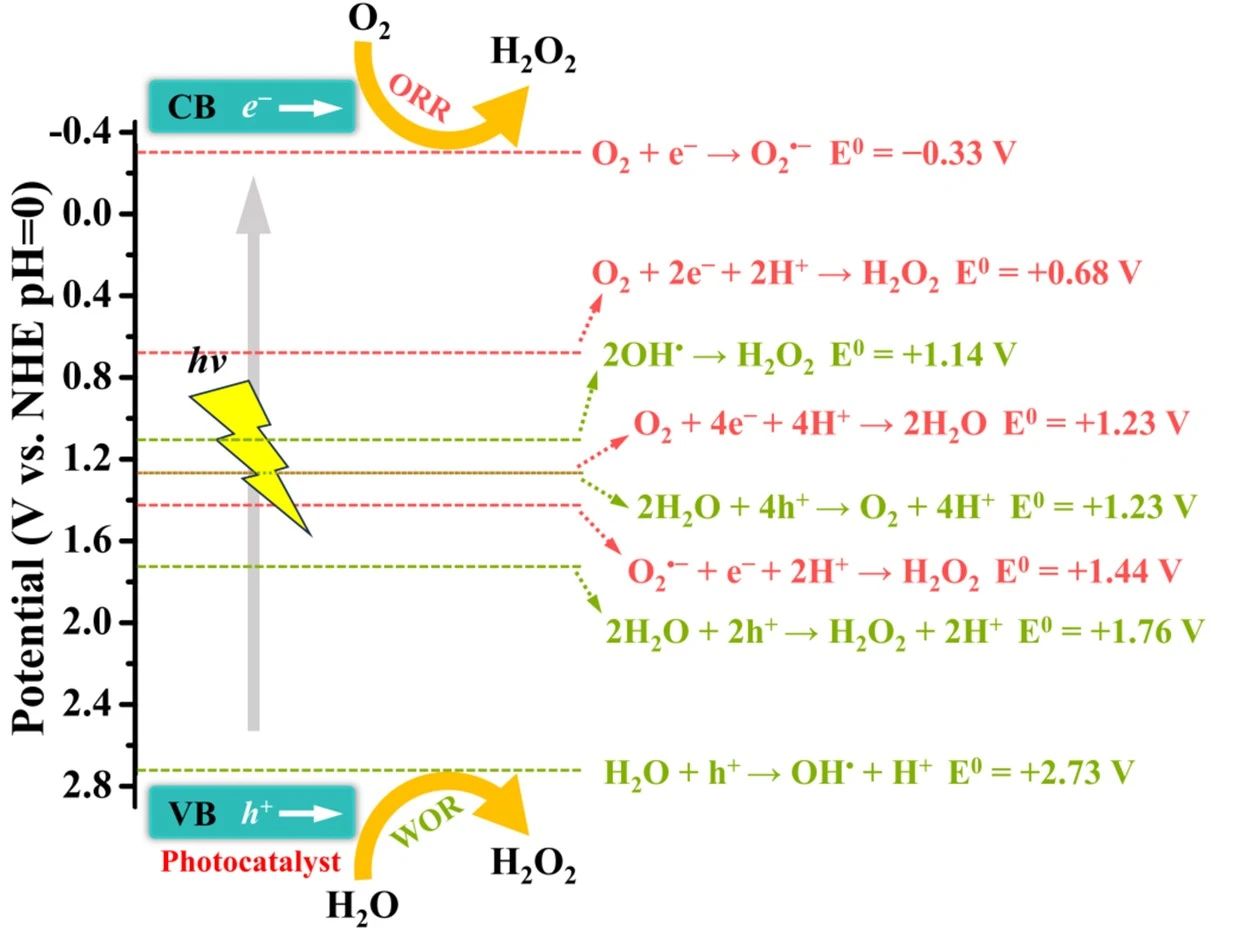
圖2.能級示意圖
可以看到H?O?合成的ORR是雙電子過程,可以將其看做直接雙電子過程或兩個單電子過程(間接雙電子過程)[8,24]。對于兩個單電子過程,O?˙?是一個重要的中間產(chǎn)物[25]。O?/H?O?的氧化還原電位(0.68 V vs. NHE)比O?/O?˙?(-0.33 V vs. NHE),根據(jù)熱力學(xué)原理,直接雙電子過程更容易發(fā)生[8]。
在光催化O?還原過程中,存在H?O?合成反應(yīng)的競爭反應(yīng),即O?可以通過光生電子進(jìn)一步還原為H?O(四電子ORR)[26-27]。O?/H?O(1.23 V vs. NHE)的氧化還原電勢比O?/H?O?的氧化還原電勢更正,從熱力學(xué)角度來看,四電子ORR更易發(fā)生[28]。通常光催化劑對氧的吸附能較低,使得產(chǎn)物易于解吸。總的來說,兩個單電子過程在動力學(xué)上更有可能發(fā)生。同時,O?極易與光生空穴發(fā)生反應(yīng),產(chǎn)生單線態(tài)氧(¹O?,O?˙?/¹O? 0.34 V vs. NHE),會導(dǎo)致H?O?產(chǎn)量降低。
總的來說,光催化H?O?合成的直接雙電子過程在熱力學(xué)上更易發(fā)生,但是在動力學(xué)觀點(diǎn)上,兩個單電子過程更有利。無論直接雙電子過程還是兩個單電子過程,都存在一些競爭反應(yīng)。因此,抑制四電子氧還原反應(yīng)和¹O?的形成,高選擇性的產(chǎn)生H?O?是光催化H?O?合成的關(guān)鍵之一。雙電子WOR與光催化H?O?合成中的雙電子ORR類似。
光催化劑在光催化H?O?氧化反應(yīng)中起著核心作用。到目前為止已經(jīng)開發(fā)出各種功能材料作為光催化劑來生產(chǎn)H?O?,并且取得了一些良好的結(jié)果[29-31]。通常為了提高光催化H?O?合成效率主要有兩種方法,即反應(yīng)條件的優(yōu)化和光催化劑的改性[32]。
No.1 反應(yīng)條件的優(yōu)化
• 溶劑類型
一般來說,合適的溶劑不僅能夠作為電子供體來捕獲空穴并提供足夠的質(zhì)子,還有助于分離光生載流子。目前最常用的溶劑是醇類,如乙醇和異丙醇。醇可以氧化成醛,產(chǎn)生質(zhì)子還原O?。Yamashita[33]等人采用樣品法制備了一種疏水性鈦摻雜鋯基MOF,這種MOF在芐醇水溶液中經(jīng)可見光照射能夠獲得很高的H?O?產(chǎn)率(9700.00 μmol L?¹ h?¹)。但是醇類的使用也會帶來的高成本和純化過程復(fù)雜問題。Lan[34]等人構(gòu)建了穩(wěn)定的鈷基金屬有機(jī)籠,金屬-非金屬活性位點(diǎn)協(xié)同作用,反應(yīng)底物可以通過籠的配位化學(xué)與活性位點(diǎn)接觸。在純水中光催化H?O?產(chǎn)生的速率高達(dá)146.60 μmol L?¹ h?¹。除此之外,采用海水光催化H?O?生產(chǎn)也是一種可行的方法。Das[35]等人合成了一種g-C?N?催化劑,這種催化劑在光催化H?O?產(chǎn)生中表現(xiàn)出優(yōu)異的活性。使用純水或海水光催化H?O?具有很好的應(yīng)用前景,但是低效率是實(shí)際應(yīng)用的最大障礙。
• pH值
反應(yīng)體系的pH值也是影響光催化H?O?生產(chǎn)的催化劑性能,很多研究工作忽略了這一點(diǎn)。Wang[36]等人制備了有機(jī)聚合物點(diǎn)(PFB-PCBM Pdots),PFBT-PCBM Pdots在堿性條件下才能實(shí)現(xiàn)光催化H?O?產(chǎn)生(圖3a)。Mao[37]等人的研發(fā)線環(huán)糊精嘧啶聚合物在酸性條件下具有更高的光催化H?O?產(chǎn)率(圖3b)。與堿性反應(yīng)環(huán)境相比,酸性反應(yīng)環(huán)境中會有更多的質(zhì)子,同時光催化劑的活性位點(diǎn)可能會受到pH值的影響,因此pH值的改變可能會對生成途徑產(chǎn)生影響。
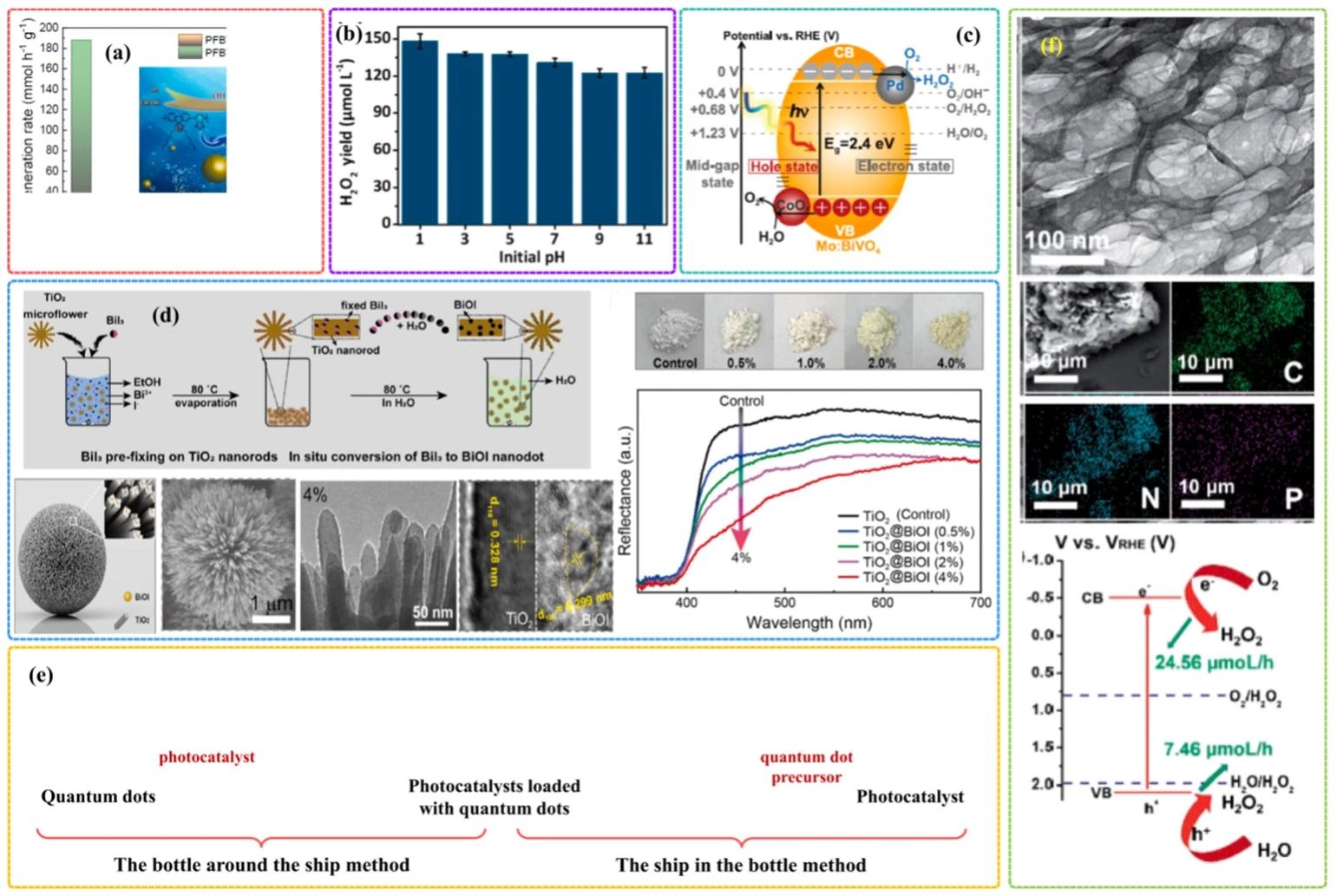
圖3.(a)pH值對光催化H?O?產(chǎn)率的影響[38];(b)pH值對光催化H?O?產(chǎn)生的影響[39];(c)光催化H?O?產(chǎn)生的示意圖[40];(d)在二氧化鈦納米棒上制備超小BiOI納米點(diǎn)的反應(yīng)物預(yù)固定策略示意圖,以及樣品的掃描電鏡、透射電鏡圖像和紫外-可見光譜[41];(e)“瓶中船”和“船中瓶”示意圖;(f)光催化劑的TEM圖像和光催化H?O?合成示意圖[42]
• O?含量
對于雙電子ORR,O?是關(guān)鍵的原料。由于水中溶解氧很少,將氧氣注入反應(yīng)系統(tǒng)是提高光催化H?O?產(chǎn)率的一種有效的方法。由空穴觸發(fā)的四電子氧化還原反應(yīng)可以為雙電子氧化還原反應(yīng)提供氧氣。Domen[43]等人的研究中,CoOx/Mo:BiVO?/Pd產(chǎn)生的空穴可以將水氧化形成氧氣,然后通過雙電子氧化還原反應(yīng)將獲得的氧氣進(jìn)一步還原為H?O?(圖3c)。
No.2 光催化劑改性
• 吸收光線的增強(qiáng)
強(qiáng)光吸收能力是光催化劑的必要屬性。一般來說,光催化劑的光吸收特性主要有能帶結(jié)構(gòu)決定。為了提高光催化劑的光吸收能力,提出了一些有效的策略來修飾光催化劑的能帶結(jié)構(gòu),例如表面改性工程、負(fù)載量子點(diǎn)、摻雜和局域表面等離子體共振(LSPR)效應(yīng)。光敏劑的引入是最常見的表面改性工程之一。Feng[44]通過在TiO?納米棒組裝的微型花朵上均勻裝飾BiOI納米點(diǎn),制備了具有代表性的納米區(qū)域光催化異質(zhì)結(jié)構(gòu)(圖3d),可見光敏化納米點(diǎn)很好的分散在TiO?納米棒表面,有效的增強(qiáng)TiO?的可見光捕獲能力。量子點(diǎn)是在納米尺度上具有獨(dú)特光學(xué)性質(zhì)的半導(dǎo)體,負(fù)載量子點(diǎn)被認(rèn)為是提高催化劑光吸收能力的可行策略。目前已報道的兩種典型的將量子點(diǎn)負(fù)載到催化劑上的方法,即“瓶中船”和“船中瓶”方法(圖3e)。Cao[45]等人采用了一種簡單的方法來構(gòu)建磷摻雜的多孔g-C?N?(圖3f),具有典型的孔結(jié)構(gòu),g-C?N?的光吸收能力也得到了提高。最高H?O?產(chǎn)率可達(dá)到1968 μmol g?¹ h?¹,光催化產(chǎn)H?O?的途徑包括雙電子ORR和雙電子WOR。
當(dāng)入射光子的頻率與金屬內(nèi)部等離子體的振蕩頻率相同時,會發(fā)生共振,導(dǎo)致入射光的強(qiáng)烈吸收,這種現(xiàn)象稱為LSPR效應(yīng)(圖4a)。Li[46]等人通過用Au納米顆粒封裝在NH?-UiO-66納米籠中,制備了Au@NH?-UiO-66/CdS復(fù)合材料,引入的金納米粒子在523 nm處表現(xiàn)出明顯的LSPR特征峰。由于金納米顆粒的LSPR效應(yīng),Au@NH?-UiO-66/CdS復(fù)合材料在析氫反應(yīng)中表現(xiàn)出優(yōu)異的催化性能,金納米粒子在波長523 nm表現(xiàn)出明顯的LSPR特征峰(圖4b),Au@NH?-UiO-66/CdS復(fù)合材料在析氫反應(yīng)中表現(xiàn)出優(yōu)異的催化性能。
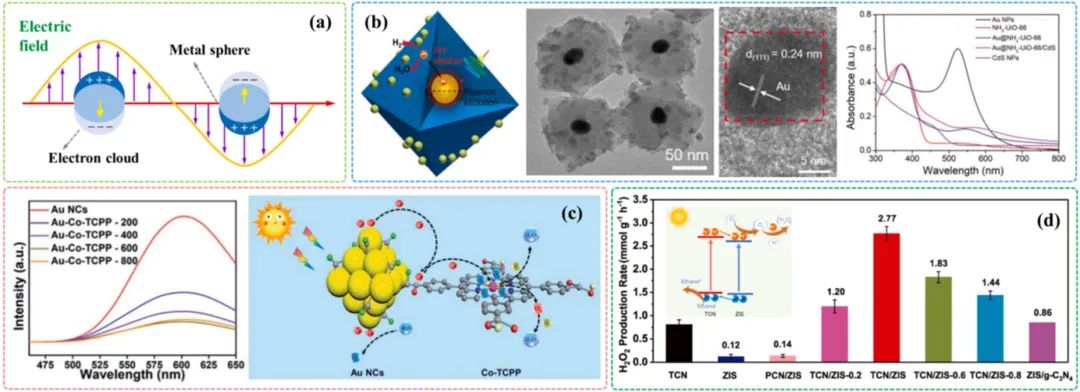
圖4.(a)表面等離子共振效應(yīng)示意圖;(b)樣品的TEM圖像和紫外可見光譜[47];(c)樣品光譜及模擬機(jī)理[48];(d)不同催化劑光催化H?O?演化比較[49]
• 電荷分離的改善
光生電子和空穴易復(fù)合是一個不可避免的問題,嚴(yán)重限制了光催化劑的工程應(yīng)用。
由于某些金屬離子具有多層價態(tài),金屬離子可通過氧化還原偶聯(lián)在電子傳遞中起一定作用。因此,摻雜金屬離子被認(rèn)為是抑制光誘導(dǎo)電子和空穴重組的有效途徑。Xue[50]等利用卟啉鈷用一種簡單的方法來裝飾金納米粒子,由于鈷離子在電子轉(zhuǎn)移中的作用,金納米粒子上的電荷分離效率明顯提高,光催化H?O?產(chǎn)率高達(dá)235.93 μmol L?¹(圖4c)。
異質(zhì)結(jié)的構(gòu)建也可以提高電荷分離效率。兩種材料結(jié)合會在界面處形成異質(zhì)結(jié),通過不同組分之間的異質(zhì)結(jié)可以實(shí)現(xiàn)光生載流子的相互傳輸。Wang[51]等人的研究中,基于原位生長方法制備了灌裝氮化碳(TCN)/ZnIn?S?(ZIS)異質(zhì)結(jié)。由于形成了II型異質(zhì)結(jié),電荷分離能力得到改善,在TCN/ZIS上可以實(shí)現(xiàn)2.77 mmol g?¹ h?¹的H?O?產(chǎn)率(圖4d)。Ye[52]等人通過耦合g-C?N?和Zn聚菲咯啉制備了一種新型的Z-scheme異質(zhì)結(jié),在純水中可以實(shí)現(xiàn)114 μmol g?¹ h?¹ H?O?的產(chǎn)率。
• 表面光催化反應(yīng)的增強(qiáng)
普遍認(rèn)為光催化H?O?合成的途徑主要涉及雙電子ORR和雙電子WOR,這些反應(yīng)通常發(fā)生在催化劑的表面,因此增強(qiáng)表面光催化反應(yīng)對光催化劑上H?O?的產(chǎn)生有積極影響。
• 抑制H?O?分解
在堿性環(huán)境或高溫下,H?O?容易分解為H?O和O?。同時在光催化劑表面形成的H?O?可以與光生電子或空穴進(jìn)一步反應(yīng)。因此及時從光催化劑表面脫附H?O?非常重要。Yamashita[53]等人將十八烷基磷酸用于對鈦摻雜的Zr基MOF進(jìn)行改性,使其具有疏水性,光催化和H?O?分散在苯基乙醇和水中,從而抑制了H?O?的分解。
太陽能驅(qū)動的光催化過程為生產(chǎn)H?O?提供了一種有前途的綠色方法。盡管過去的幾十年,許多研究人員致力于探索光催化H?O?產(chǎn)生在工程應(yīng)用的可行性。盡管取得了顯著的進(jìn)展,光催化H?O?生產(chǎn)仍有很長的路要走。眾所周知,光催化H?O?合成的核心是光催化劑,然而目前大多數(shù)光催化劑還沒有強(qiáng)光吸收能力和高電子-空穴對分離效率,未來研究可以繼續(xù)關(guān)注這些問題。此外,表面光催化反應(yīng)的改善對光催化劑H?O?合成具有積極影響。除上述問題外,缺乏合適的反應(yīng)器,使得光催化劑的回收和再生困難,限制了光催化H?O?合成在實(shí)際工程中的應(yīng)用。雖然有許多挑戰(zhàn)和問題阻礙光催化H?O?合成進(jìn)一步發(fā)展,但光催化H?O?合成仍然是一個有吸引力的領(lǐng)域。
參考文獻(xiàn):
[1] R.H. Adnan, A.A. Jalil, Gold photocatalysis in sustainable hydrogen peroxide generation, Mater. Today Chem. 27 (2023) 101322.
[2] W. Zhao, P. Yan, B. Li, M. Bahri, L. Liu, X. Zhou, R. Clowes, N.D. Browning, Y. Wu, J.W. Ward, A.I. Cooper, Accelerated Synthesis and Discovery of Covalent Organic Framework Photocatalysts for Hydrogen Peroxide Production, J. Am. Chem. Soc. 144 (2022) 9902–9909.
[3] R. Dhawle, J. Vakros, V. Dracopoulos, I.D. Manariotis, D. Mantzavinos, P. Lianos, Enhancement of the photoelectrochemical production of hydrogen peroxide under intermittent light supply in the presence of an optimized biochar supercapacitor, Electrochim. Acta 427 (2022) 140846.
[4] Q. Hu, Y. Huang, X. Yu, S. Gong, Y. Wen, Y. Liu, G. Li, Q. Zhang, R. Ye, X. Chen, Ultrafast Hole Transfer in Graphitic Carbon Nitride Imide Enabling Efficient H?O? Photoproduction, ACS Appl. Mater. Interfaces 15 (2023) 42611–42621.
[5] F. Hao, C. Yang, X. Lv, F. Chen, S. Wang, G. Zheng, Q. Han, Photo-Driven QuasiTopological Transformation Exposing Highly Active Nitrogen Cation Sites for Enhanced Photocatalytic H?O? Production. Angewandte Chemie - International Edition (2023) 2315456.
[6] A. Rogolino, I.F. Silva, N.V. Tarakina, M.A.R. Da Silva, G.F.S.R. Rocha, M. Antonietti, I.F. Teixeira, Modified Poly (Heptazine Imides): Minimizing H?O? Decomposition to Maximize Oxygen Reduction, ACS Appl. Mater. (2022) 49820–49829.
[7] D. Chen, W. Chen, Y. Wu, L. Wang, X. Wu, H. Xu, L. Chen, Covalent Organic Frameworks Containing Dual O? Reduction Centers for Overall Photosynthetic Hydrogen Peroxide Production, Angew. Chem. Int. Ed. 2217479 (2023).
[8] Y. Cong, X. Li, S. Zhang, Q. Zheng, Y. Zhang, S. Lv, Embedding Carbon Quantum Dots into Crystalline Polyimide Covalent Organic Frameworks to Enhance Water Oxidation for Achieving Dual-Channel Photocatalytic H?O? Generation in a Wide pH Range, ACS Appl. Mater. Interfaces 15 (2023) 43799–43809.
[9] Y. Zhao, J. Gao, Z. Yang, L. Li, J. Cui, P. Zhang, C. Hu, C. Diao, W. Choi, Efficient Exciton Dissociation in Ionically Interacted Methyl Viologen and Polymeric Carbon Nitride for Superior H?O? Photoproduction, ACS Catal. 5 (2023) 2790–2801.
[10] S. Chai, X. Chen, X. Zhang, Y. Fang, R.S. Sprick, X. Chen, Rational design of covalent organic frameworks for efficient photocatalytic hydrogen peroxide production, Environ. Sci.-Nano 9 (2022) 2464–2469.
[11] Y. Cong, S. Zhang, Q. Zheng, X. Li, Y. Zhang, S. Lv, Oxygen-modified graphitic carbon nitride with nitrogen-defect for metal-free visible light photocatalytic H?O? evolution, J. Colloid Interface Sci. 650 (2023) 1013–1021.
[12] C. Zhuang, W. Li, T. Zhang, J. Li, Y. Zhang, G. Chen, H. Li, Z. Kang, J. Zou, X. Han, Monodispersed aluminum in carbon nitride creates highly efficient nitrogen active sites for ultra-high hydrogen peroxide photoproduction, Nano Energy 108 (2023) 108225.
[13] J. Chen, Q. Ma, X. Zheng, Y. Fang, J. Wang, S. Dong, Kinetically restrained oxygen reduction to hydrogen peroxide with nearly 100% selectivity, Nat. Commun. 13(2022)7457.
[14] Q. Liao, Q. Sun, H. Xu, Y. Wang, Y. Xu, Z. Li, J. Hu, D. Wang, H. Li, K. Xi, Regulating Relative Nitrogen Locations of Diazine Functionalized Covalent Organic Frameworks for Overall H?O? Photosynthesis, Angew. Chem. – Int. Ed. 62 (2023) 2310556.
[15] R.A. Borges, M.F. Pedrosa, Y.A. Manrique, C.G. Silva, A.M.T. Silva, J.L. Faria, M. J. Sampaio, 3D structured photocatalysts for sustainable H?O? generation from saccharides derivatives, Chem. Eng. J. 470 (2023) 144066.
[16] Q. Hu, Y. Dong, K. Ma, X. Meng, Y. Ding, Amidation crosslinking of polymeric carbon nitride for boosting photocatalytic hydrogen peroxide production, J. Catal. 413 (2022) 321–330.
[17] Y. Zhao, L. Xu, X. Wang, Z. Wang, Y. Liu, Y. Wang, Q. Wang, Z. Wang, H. Huang, Y. Liu, W. Wong, Z. Kang, A comprehensive understanding on the roles of carbon dots in metallated graphyne based catalyst for photoinduced H?O? production, Nano Today 43 (2022) 101428.
[18] Y. Guo, X. Tong, N. Yang, Photocatalytic and Electrocatalytic Generation of Hydrogen Peroxide: Principles, Catalyst Design and Performance. Nano-Micro Lett. 15 (2023) 77.
[19] S. Lv, Q. Zheng, L. Ye, C. Li, J. Liu, Y. Cong, S. Wang, The elaborately-designed Zscheme Fe-g-C?N?/α-Fe?O? photocatalytic platform equipping with active N-Fe-O bridges for enhanced synergistic removal of tetracycline and Cr (VI) via photoinduced electron transfer process, Chem. Eng. J. 455 (2023) 140940.
[20] Y. Ding, S. Maitra, S. Halder, C. Wang, R. Zheng, T. Barakat, S. Roy, L. Chen, B. Su, Emerging semiconductors and metal-organic-compounds-related photocatalysts for sustainable hydrogen peroxide production, Matter 5 (2022) 2119–2167.
[21] J. Sun, J. Chakraborty, M. Deng, A. Laemont, X. Feng, Y. Liu, P. van der Voort, Metal-organic frameworks and covalent organic frameworks as photocatalysts for H?O? production from oxygen and water, J. Mater. Chem. A 11 (2023) 21516–21540.
[22] Y. Zhang, C. Pan, G. Bian, J. Xu, Y. Dong, Y. Zhang, Y. Lou, W. Liu, Y. Zhu, H?O? generation from O? and H?O on a near-infrared absorbing porphyrin supramolecular photocatalyst, Nat. Energy 8 (2023) 361–371.
[23] B. Liu, W. Zhang, Q. Zhang, Y. Guan, Z. Lu, Synergistic Promotion of the Photocatalytic Preparation of Hydrogen Peroxide (H?O?) from Oxygen by Benzoxazine and SiOTi Bond, Small 2303907 (2023).
[24] L. Zhai, Z. Xie, C. Cui, X. Yang, Q. Xu, X. Ke, M. Liu, L. Qu, X. Chen, L. Mi, Constructing Synergistic Triazine and Acetylene Cores in Fully Conjugated Covalent Organic Frameworks for Cascade Photocatalytic H?O? Production, Chem. Mat. 34 (2022) 5232–5240.
[25] X. Zhang, P. Ma, C. Wang, L. Gan, X. Chen, P. Zhang, Y. Wang, H. Li, L. Wang, X. Zhou, K. Zheng, Unraveling the dual defect sites in graphite carbon nitride for ultra-high photocatalytic H?O? evolution, Energy Environ. Sci. 15 (2022) 830–842.
[26] X. Zhang, H. Su, P. Cui, Y. Cao, Z. Teng, Q. Zhang, Y. Wang, Y. Feng, R. Feng, J. Hou, X. Zhou, P. Ma, H. Hu, K. Wang, C. Wang, L. Gan, Y. Zhao, Q. Liu, T. Zhang, K. Zheng, Developing Ni single-atom sites in carbon nitride for efficient photocatalytic H?O? production, Nat. Commun. 14 (2023) 7115.
[27] B. Liu, J. Du, G. Ke, B. Jia, Y. Huang, H. He, Y. Zhou, Z. Zou, Boosting O? Reduction and H?O Dehydrogenation Kinetics: Surface N-Hydroxymethylation of g-C?N? Photocatalysts for the Efficient Production of H?O?, Adv. Funct. Mater. 32 (2022) 2111125.
[28] S. Qu, H. Wu, Y.H. Ng, Clean Production of Hydrogen Peroxide: A Heterogeneous Solar-Driven Redox Process, Adv. Energy Mater. 13 (2023) 2301047.
[29] M. Deng, J. Sun, A. Laemont, C. Liu, L. Wang, L. Bourda, J. Chakraborty, K. Van Hecke, R. Morent, N. De Geyter, K. Leus, H. Chen, P. van der Voort, Extending the π-conjugation system of covalent organic frameworks for more efficient photocatalytic H?O? production, Green Chem. 25 (2023) 3069–3076.
[30] K. Jinguji, M. Watanabe, R. Morita, Y. Takaoka, M.S. Hossain, J.T. Song, A. Takagaki, J. Matsuda, T. Ishihara, Visible light driven hydrogen peroxide production by oxygen and phosphorus co-doped CoP-C?N? photocatalyst, Catal. Today 426 (2024) 114400.
[31] M. Ikram, A. Raza, S.O.A. Ahmad, A. Ashfaq, M.U. Akbar, M. Imran, S. Dilpazir, M. Khan, Q. Khan, M. Maqbool, Solar-Triggered Engineered 2D-Materials for Environmental Remediation: Status and Future Insights, Adv. Mater. Interfaces 10 (2023) 2202172.
[32] Y. Kondo, Y. Kuwahara, K. Mori, H. Yamashita, Design of metal-organic framework catalysts for photocatalytic hydrogen peroxide production, Chem 8 (2022) 2924–2938.
[33] X. Chen, Y. Kuwahara, K. Mori, C. Louis, H. Yamashita, A hydrophobic titanium doped zirconium-based metal organic framework for photocatalytic hydrogen peroxide production in a two-phase system, J. Mater. Chem. A 8 (2020) 1904–1910.
[34] J. Lu, J. Liu, L. Dong, J. Lin, F. Yu, J. Liu, Y. Lan, Synergistic Metal-Nonmetal Active Sites in a Metal-Organic Cage for Efficient Photocatalytic Synthesis of Hydrogen Peroxide in Pure Water, Angewandte Chemie - International Edition 62 (2023) 2308505.
[35] P. Ren, T. Zhang, N. Jain, H.Y.V. Ching, A. Jaworski, G. Barcaro, S. Monti, J. Silvestre-Albero, V. Celorrio, L. Chouhan, A. Rokicinska, E. Debroye, P. Kustrowski, S. Van Doorslaer, S. Van Aert, S. Bals, S. Das, An Atomically Dispersed Mn-Photocatalyst for Generating Hydrogen Peroxide from Seawater via the Water Oxidation Reaction (WOR), J. Am. Chem. Soc. 145 (2023) 16584–16596.
[36] S. Wang, B. Cai, H. Tian, Efficient Generation of Hydrogen Peroxide and Formate by an Organic Polymer Dots Photocatalyst in Alkaline Conditions, Angewandte Chemie - International Edition 61 (2022) 2202733.
[37] C. Chu, Q. Li, W. Miao, H. Qin, X. Liu, D. Yao, S. Mao, Photocatalytic H?O? production driven by cyclodextrin-pyrimidine polymer in a wide pH range without electron donor or oxygen aeration, Applied Catalysis b: Environmental 314 (2022) 121485.
[38] Z. Zheng, F. Han, B. Xing, X. Han, B. Li, Synthesis of Fe?O?@CdS@CQDs ternary core-shell heterostructures as a magnetically recoverable photocatalyst for selective alcohol oxidation coupled with H?O? production, J. Colloid Interface Sci. 624 (2022) 460–470.
[39] X. Li, Q. Zheng, X. Wang, Q. Zheng, Y. Zhang, Y. Cong, S. Lv, Introduction of electron-deficient unit in resorcinol-formaldehyde resin to construct donor–acceptor conjugated polymer for enhancing photocatalytic H?O? production, J. Mater. Chem. A 12 (2024) 8420–8428.
[40] Y. Shiraishi, M. Matsumoto, S. Ichikawa, S. Tanaka, T. Hirai, Polythiophene-Doped Resorcinol-Formaldehyde Resin Photocatalysts for Solar-to-Hydrogen Peroxide Energy Conversion, J. Am. Chem. Soc. 143 (2021) 12590–12599.
[41] J. Chang, Q. Li, J. Shi, M. Zhang, L. Zhang, S. Li, Y. Chen, S. Li, Y. Lan, OxidationReduction Molecular Junction Covalent Organic Frameworks for Full Reaction Photosynthesis of H?O?, Angewandte Chemie - International Edition 62 (2023) 2218868.
[42] Y. Kondo, Y. Kuwahara, K. Mori, H. Yamashita, Design of metal-organic framework catalysts for photocatalytic hydrogen peroxide production, Chem 8 (2022) 2924–2938.
[43] T. Liu, Z. Pan, J.J.M. Vequizo, K. Kato, B. Wu, A. Yamakata, K. Katayama, B. Chen, C. Chu, K. Domen, Overall photosynthesis of H?O? by an inorganic Overall photosynthesis of H?O? by an inorganic semiconductor. Nat. Commun. 13 (2022) 1034.
[44] H. Feng, L. Liang, Y. Liu, Z. Huang, L. Li, Efficient nano-regional photocatalytic heterostructure design via the manipulation of reaction site self-quenching effect, Applied Catalysis b: Environmental 243 (2019) 220–228.
[45] J. Cao, H. Wang, Y. Zhao, Y. Liu, Q. Wu, H. Huang, M. Shao, Y. Liu, Z. Kang, Phosphorus-doped porous carbon nitride for efficient sole production of hydrogen peroxide via photocatalytic water splitting with a two-channel pathway, J. Mater. Chem. A 8 (2020) 3701–3707.
[46] Z. Li, J. Zi, X. Luan, Y. Zhong, M. Qu, Y. Wang, Z. Lian, Localized Surface Plasmon Resonance Promotes Metal-Organic Framework-Based Photocatalytic Hydrogen Evolution, Adv. Funct. Mater. 33 (2023) 2303069.
[47] P. Ren, T. Zhang, N. Jain, H.Y.V. Ching, A. Jaworski, G. Barcaro, S. Monti, J. Silvestre-Albero, V. Celorrio, L. Chouhan, A. Rokicinska, E. Debroye, P. Kustrowski, S. Van Doorslaer, S. Van Aert, S. Bals, S. Das, An Atomically Dispersed Mn-Photocatalyst for Generating Hydrogen Peroxide from Seawater via the Water Oxidation Reaction (WOR), J. Am. Chem. Soc. 145 (2023) 16584–16596.
[48] S. Wang, B. Cai, H. Tian, Efficient Generation of Hydrogen Peroxide and Formate by an Organic Polymer Dots Photocatalyst in Alkaline Conditions, Angewandte Chemie - International Edition 61 (2022) 2202733.
[49] C. Chu, Q. Li, W. Miao, H. Qin, X. Liu, D. Yao, S. Mao, Photocatalytic H?O? production driven by cyclodextrin-pyrimidine polymer in a wide pH range without electron donor or oxygen aeration, Applied Catalysis b: Environmental 314 (2022) 121485.
[50] Q. Xue, Z. Wang, S. Han, Y. Liu, X. Dou, Y. Li, H. Zhu, X. Yuan, Ligand engineering of Au nanoclusters with multifunctional metalloporphyrins for photocatalytic H?O? production, J. Mater. Chem. A 10 (2022) 8371–8377.
[51] J. Wang, C. Guo, Y. Jiang, J. Wan, B. Zheng, Y. Li, B. Jiang, Highly efficient photocatalytic H?O? production by tubular g-C?N?/ZnIn?S? nanosheet heterojunctions via improved charge separation, Sci. China-Mater. 66 (2023) 1053–1061.
[52] Y. Ye, J. Pan, F. Xie, L. Gong, S. Huang, Z. Ke, F. Zhu, J. Xu, G. Ouyang, Highly efficient photosynthesis of hydrogen peroxide in ambient conditions, Proc. Natl. Acad. Sci. u. s. a. 118 (2021).
[53] X. Chen, Y. Kuwahara, K. Mori, C. Louis, H. Yamashita, A hydrophobic titanium doped zirconium-based metal organic framework for photocatalytic hydrogen peroxide production in a two-phase system, J. Mater. Chem. A 8 (2020) 1904–1910.